Nur77 modulator 1
CAS No. 2469975-55-9
Nur77 modulator 1( —— )
Catalog No. M28278 CAS No. 2469975-55-9
Nur77 modulator 1 is a good Nur77 binder (K D = 3.58 μM). Nur77 modulator 1 up-regulates Nur77 expression, mediates sub-cellular localization of Nur77, induces Nur77-dependent ER stress and autophagy, and results in cell apoptosis. Anti-hepatoma activity .
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 259 | Get Quote |


|
| 10MG | 430 | Get Quote |


|
| 25MG | 710 | Get Quote |


|
| 50MG | 972 | Get Quote |


|
| 100MG | 1332 | Get Quote |


|
| 500MG | 2673 | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameNur77 modulator 1
-
NoteResearch use only, not for human use.
-
Brief DescriptionNur77 modulator 1 is a good Nur77 binder (K D = 3.58 μM). Nur77 modulator 1 up-regulates Nur77 expression, mediates sub-cellular localization of Nur77, induces Nur77-dependent ER stress and autophagy, and results in cell apoptosis. Anti-hepatoma activity .
-
DescriptionNur77 modulator 1 is a good Nur77 binder (K D = 3.58 μM). Nur77 modulator 1 up-regulates Nur77 expression, mediates sub-cellular localization of Nur77, induces Nur77-dependent ER stress and autophagy, and results in cell apoptosis. Anti-hepatoma activity .(In Vitro):Nur77 modulator 1 (10g, 0-20 μM) displays potent and broad-spectrum antiproliferative activity against all tested three hepatoma cell lines (HepG2, QGY-7703, and SMMC-7721), and less cytotoxicity against LO2 cells (human normal liver cell line) . Nur77 modulator 1 (10g, ) could specifically up-regulate the expression of Nur77 in a dose-dependent manner . Nur77 modulator 1 (10g, 2.0 μM) induces Nur77-dependent apoptosis . Nur77 modulator 1 (10g, 2.0 μM) increased LC3-II and Beclin1 protein levels in a dose-dependent manner (10g treatment does not enhance p62 degradation, indicating that autophagy induced by 10g is incomplete) . Cell Viability Assay . Cell Line: Liver cancer cell lines (HepG2, QGY-7703, and SMMC-7721). Concentration: 0-20 μM. Incubation Time: 12-24 hours. Result: Exhibited IC 50 values of 0.6 μM, 0.89 μM, 1.40 μM and >20 μM in HepG2, QGY-7703, SMMC-7721 and LO2 cells, respectively. Reduced the viability in a time-dependent manner. Induced cell morphology alteration, such as cell shrinkage, vesicles accumulated, and reduced cell number.(In Vivo):Nur77 modulator 1 (10g, 10 and 20 mg/kg/day) exhibits significant anti-tumor activity in mouse hepatoma HepG2 xenograft with good tolerability . Animal Model: Nude mouse hepatoma HepG2 xenograft . Dosage: 10 and 20 mg/kg/day. Administration: IP, once every day for 15 days. Result: Lead to substantial suppression of tumor growth. The tumor growth inhibition (TGI) values at doses of 10mg/kg/day and 20 mg/kg/day were 36.74 % and 62.38 %, respectively. Exhibited almost no influence on the body weight of experimental mice.
-
In VitroNur77 modulator 1 (10g, 0-20 μM) displays potent and broad-spectrum antiproliferative activity against all tested three hepatoma cell lines (HepG2, QGY-7703, and SMMC-7721), and less cytotoxicity against LO2 cells (human normal liver cell line).Nur77 modulator 1 (10g, ) could specifically up-regulate the expression of Nur77 in a dose-dependent manner.Nur77 modulator 1 (10g, 2.0 μM) induces Nur77-dependent apoptosis.Nur77 modulator 1 (10g, 2.0 μM) increased LC3-II and Beclin1 protein levels in a dose-dependent manner (10g treatment does not enhance p62 degradation, indicating that autophagy induced by 10g is incomplete). Cell Viability Assay.Cell Line:Liver cancer cell lines (HepG2, QGY-7703, and SMMC-7721).Concentration:0-20 μM.Incubation Time:12-24 hours.Result:Exhibited IC50 values of 0.6 μM, 0.89 μM, 1.40 μM and >20 μM in HepG2, QGY-7703, SMMC-7721 and LO2 cells, respectively.Reduced the viability in a time-dependent manner.Induced cell morphology alteration, such as cell shrinkage, vesicles accumulated, and reduced cell number.
-
In VivoNur77 modulator 1 (10g, 10 and 20 mg/kg/day) exhibits significant anti-tumor activity in mouse hepatoma HepG2 xenograft with good tolerability. Animal Model:Nude mouse hepatoma HepG2 xenograft.Dosage:10 and 20 mg/kg/day.Administration:IP, once every day for 15 days.Result:Lead to substantial suppression of tumor growth.The tumor growth inhibition (TGI) values at doses of 10mg/kg/day and 20 mg/kg/day were 36.74 % and 62.38 %, respectively.Exhibited almost no influence on the body weight of experimental mice.
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorIGF-1R|Akt|mTOR
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number2469975-55-9
-
Formula Weight495.6
-
Molecular FormulaC28H25N5O2S
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 125 mg/mL (252.22 mM)
-
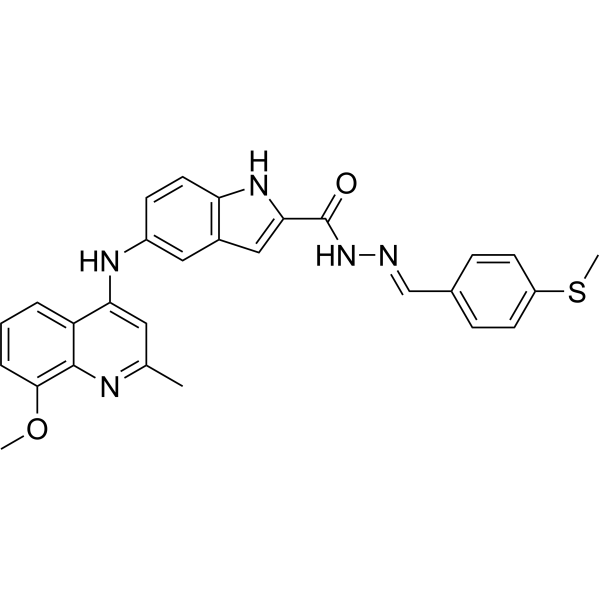
SMILESCOC1=CC=CC2=C1N=C(C)C=C2NC3=CC=C(NC(C(N/N=C/C4=CC=C(SC)C=C4)=O)=C5)C5=C3
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Seol HS, et al. Loss of miR-100 and miR-125b results in cancer stem cell properties through IGF2 upregulation in hepatocellular carcinoma. Sci Rep. 2020 Dec 8;10(1):21412.
molnova catalog



related products
-
PEN-221
PEN-221 is a Somatostatin receptor 2 (SSTR2)-targeting cytotoxic conjugate with an IC50 of 10 nM. PEN-221 is a conjugate consisting of microtubule-targeting agent DM1 linked to the C-terminal side chain of Tyr3-octreotate.
-
IMR-1
IMR-1 is a novel class of Notch inhibitors targeting the transcriptional activation with IC50 of 6 μMol/L.
-
N-Acetyl-L-aspartic ...
N-Acetylaspartic acid is a derivative of aspartic acid. It is the second most concentrated molecule in the brain after the amino acid glutamate. It is synthesized in neurons from the amino acid aspartate and acetyl coenzyme A (acetyl CoA).



 Cart
Cart
 sales@molnova.com
sales@molnova.com


